Schwann cells are a special type of cell found in the peripheral nervous system (PNS). They play a key role in helping nerves work efficiently by wrapping around nerve fibers and forming a protective layer called the myelin sheath.
This insulation allows electrical signals to travel quickly and smoothly along the nerves, which is essential for everything from moving your muscles to sensing temperature.

Key Takeaways
- Schwann cells are glial cells in the peripheral nervous system that insulate, support, and repair nerve fibers.
- They come in two types: myelinating (which wrap axons in myelin) and non-myelinating (which support small nerve fibers).
- These cells are vital for nerve signal speed, protection, and regeneration after injury.
- Damage to Schwann cells can cause or contribute to several neurological disorders.
- Their unique functions make them an exciting target for regenerative medicine and nerve repair research.
Did you know? Schwann cells are named after Theodor Schwann, a German physiologist who discovered these types of cells in the 19th century.
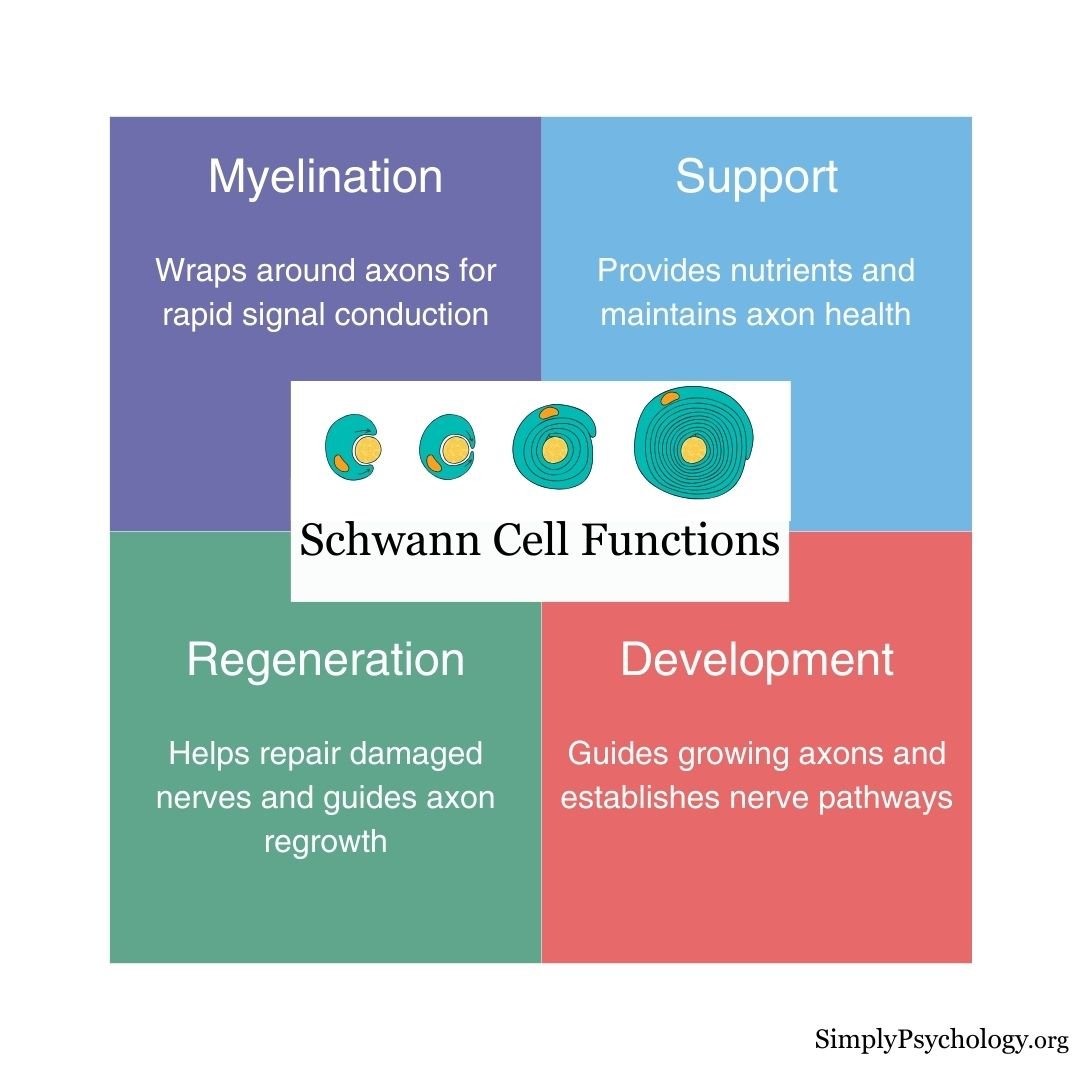
Function
Schwann cells have several important functions:
- Myelination: They create the myelin sheath, a fatty layer that insulates axons (the long parts of nerve cells) to speed up signal transmission.
- Support and protection: They supply nutrients, remove cellular waste, and shield nerve fibers from damage.
- Repair and regeneration: After injury, Schwann cells help clean up debris and guide new nerve growth by forming pathways and releasing growth factors.
- Development and communication: They help nerves develop correctly in the embryo and maintain healthy communication with neurons throughout life.
Schwann cells are also considered to be a type of glial cell. Glia cells are non-neuronal cells that do not provide electrical impulses like neurons but function to maintain homeostasis, providing support and protection for neurons. So, although Schwann cells do not conduct electrical activity themselves, they still help ensure the normal conduction of electrical signals.
Myelinating vs. Non-Myelinating Schwann Cells
There are two main types of Schwann cells:
- Myelinating Schwann cells wrap around one axon segment, creating a myelin sheath that increases the speed of electrical signals.
- Non-myelinating Schwann cells support small nerve fibers without forming myelin. They help organize and protect groups of unmyelinated axons.
Both types are essential for keeping peripheral nerves healthy and functioning.
How Schwann Cells Form Myelin
The process of myelination begins during fetal development. Schwann cells wrap themselves around a single axon in a spiral, forming multiple layers.
The inner layers become compacted to form the insulating myelin sheath, while the outermost layer remains as the cell body (called the neurilemma), which supports and maintains the sheath.
This insulation allows electrical signals to jump quickly between gaps in the myelin (called nodes of Ranvier), speeding up communication across long distances.
Schwann Cells vs. Other Glial Cells
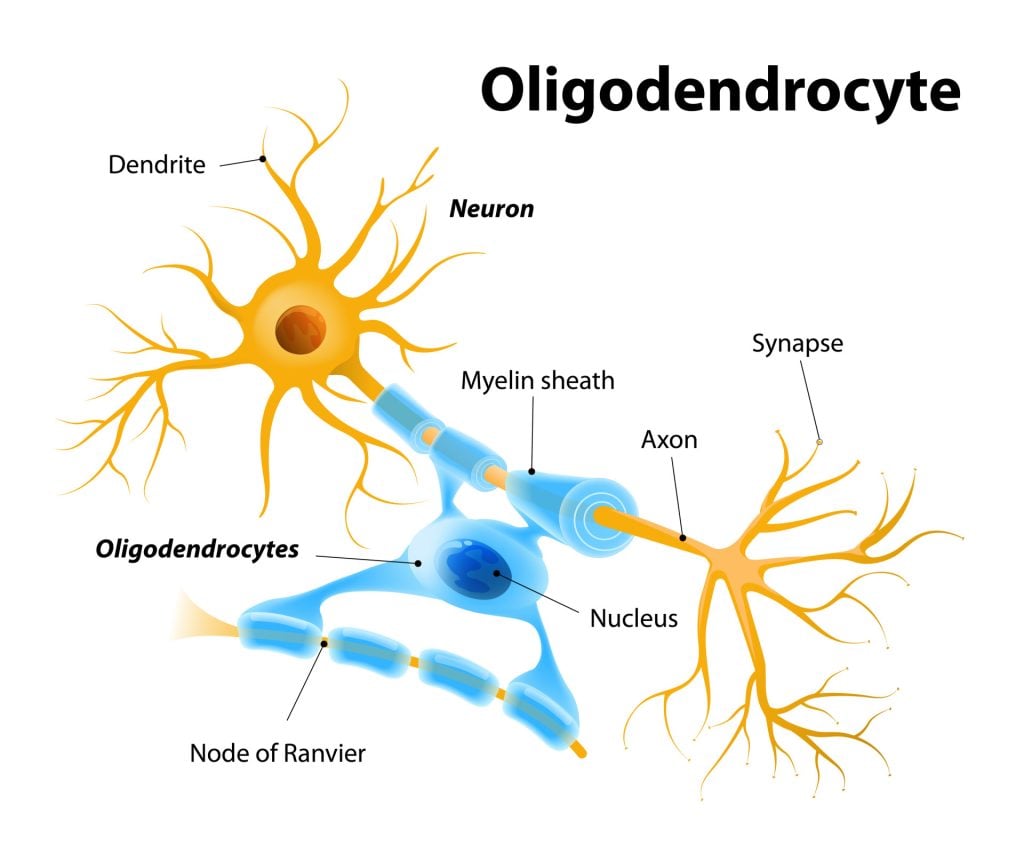
Schwann cells are the principal glial cells of the peripheral nervous system (PNS), primarily responsible for forming myelin sheaths around axons to facilitate rapid nerve impulse conduction.
In contrast, the central nervous system (CNS) contains several distinct types of glial cells with specialized functions:
- Oligodendrocytes: These cells myelinate axons in the CNS. Unlike Schwann cells, which myelinate a single axon segment, a single oligodendrocyte can extend its processes to myelinate multiple axons simultaneously.
- Astrocytes: Star-shaped cells that maintain the blood-brain barrier, regulate ion concentrations, and provide metabolic support to neurons. They also play a role in repairing the CNS after injury.
- Microglia: These act as the immune cells of the CNS, constantly surveying the environment for pathogens or damaged neurons and clearing debris through phagocytosis.
- Ependymal Cells: They line the ventricles of the brain and the central canal of the spinal cord, playing a crucial role in the production and circulation of cerebrospinal fluid.
While Schwann cells and oligodendrocytes share the function of myelination, their anatomical locations and capacities differ significantly.
Moreover, the CNS glial cells exhibit a broader range of functions beyond myelination, highlighting the specialized roles of glial cells across the nervous system.
How do Schwann cells respond to damaged axons?
When a peripheral nerve is injured, Schwann cells take action:
- They clean up damaged myelin and axon fragments.
- They release chemical signals that attract immune cells to help with cleanup.
- They produce growth-promoting substances like neurotrophins.
- They form a tunnel-like structure to guide regrowing axons back to their target.
Without Schwann cells, nerve regeneration in the PNS would be slow or impossible.
Damage
Schwann cell damage is associated with demyelinating diseases of the PNS.
Damage to Schwann cells can occur due to various factors, including genetic mutations, autoimmune responses, infections, and trauma.
When Schwann cells are damaged, the myelin sheath that insulates and supports axons can be destroyed, leading to impaired nerve conduction and potential neurodegeneration.
Signs of damaged Schwann cells include:
- Weakened reflexes
- Muscle weakness
- Sensory loss
- Slower nerve conduction
- Paralysis
What Diseases Involve Schwann Cells?
Damage to Schwann cells or their myelin can lead to serious nerve disorders:
- Guillain-Barré Syndrome (GBS): An autoimmune disease that attacks Schwann cells, causing weakness and paralysis.
- Charcot-Marie-Tooth Disease (CMT): A genetic disorder affecting Schwann cell structure, leading to muscle weakness and loss of sensation.
- Diabetic Neuropathy: High blood sugar damages Schwann cells over time, leading to numbness, pain, and mobility issues.
- Schwannomas: Usually benign tumors that grow from Schwann cells, sometimes affecting nearby nerves.
Schwann cells from the PNS have been implanted into the central nervous system (CNS) to treat multiple sclerosis-type damage and spinal cord injuries in animal studies. These implanted cells have shown promise in promoting axonal regeneration and myelination, leading to improved nerve impulse conduction (Kohama et al., 2001).
References
Britannica, T. Editors of Encyclopaedia (2020, July 15). Schwann cell. Encyclopedia Britannica. https://www.britannica.com/science/Schwann-cell
Fallon, M., & Tadi, P. (2019). Histology, Schwann Cells.
Kohama, I., Lankford, K. L., Preiningerova, J., White, F. A., Vollmer, T. L., & Kocsis, J. D. (2001). Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. Journal of Neuroscience, 21 (3), 944-950.
Oudega, M., & Xu, X. M. (2006). Schwann cell transplantation for repair of the adult spinal cord. Journal of neurotrauma, 23 (3-4), 453-467.
Sinha Dutta, S. (2020, February 4). What are Schwann Cells? News Medical Life Sciences. https://www.news-medical.net/health/What-are-Schwann-Cells.aspx#2

